Nitrous gauge Question?!!?
#5
Guest
Posts: n/a
It will peg the gauge needle. Dont know if will cause any damage to it.
The pressure shouldn't get that high when filling the bottle unless the mother bottle is almost empty and they are just pumping away to get it filled. Then is might heat the bottle up to those pressures.
-Chris
The pressure shouldn't get that high when filling the bottle unless the mother bottle is almost empty and they are just pumping away to get it filled. Then is might heat the bottle up to those pressures.
-Chris
Trending Topics
#9
TECH Enthusiast
Thread Starter
iTrader: (21)
Join Date: Nov 2008
Posts: 633
Likes: 0
Received 0 Likes
on
0 Posts
K thxs alot i dont plan on heating the bottle up over 1100 max but was consered about it sitting in the sun and haven the weak point being my gauge and if that was the case i would probly get rid of that gauge and get a bigger max point one
#11
TECH Apprentice
Join Date: Nov 2006
Location: Copenhagen
Posts: 369
Likes: 0
Received 0 Likes
on
0 Posts
look at the diagram below.. at no point will increasing pressure turn any liquid to a gas ...
Gas PropertiesMolecular WeightMolecular weight : 44.013 g/mol
Solid phaseMelting point : -91 °C
Latent heat of fusion (1,013 bar, at triple point) : 148.53 kJ/kg
Liquid phaseLiquid density (1.013 bar at boiling point) : 1222.8 kg/m3
Liquid/gas equivalent (1.013 bar and 15 °C (59 °F)) : 662 vol/vol
Boiling point (1.013 bar) : -88.5 °C
Latent heat of vaporization (1.013 bar at boiling point) : 376.14 kJ/kg
Vapor pressure (at 20 °C or 68 °F) : 58.5 bar
Critical pointCritical temperature : 36.4 °C
Critical pressure : 72.45 bar
Gaseous phaseGas density (1.013 bar at boiling point) : 3.16 kg/m3
Gas density (1.013 bar and 15 °C (59 °F)) : 1.872 kg/m3
Compressibility Factor (Z) (1.013 bar and 15 °C (59 °F)) : 0.9939
Specific gravity (air = 1) (1.013 bar and 21 °C (70 °F)) : 1.53
Specific volume (1.013 bar and 21 °C (70 °F)) : 0.543 m3/kg
Heat capacity at constant pressure (Cp) (1.013 bar and 15 °C (59 °F)) : 0.038 kJ/(mol.K)
Heat capacity at constant volume (Cv) (1.013 bar and 15 °C (59 °F)) : 0.029 kJ/(mol.K)
Ratio of specific heats (Gamma:Cp/Cv) (1.013 bar and 15 °C (59 °F)) : 1.302256
Viscosity (1.013 bar and 0 °C (32 °F)) : 0.000136 Poise
Thermal conductivity (1.013 bar and 0 °C (32 °F)) : 14.57 mW/(m.K)
MiscellaneousSolubility in water (1.013 bar and 5 °C (41 °F)) : 1.14 vol/vol
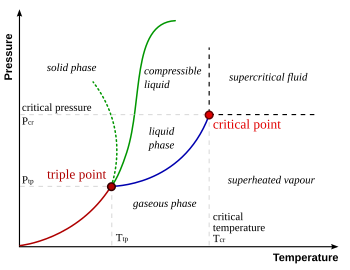
the dotted green line is the funky solid to fluid line of water.. without wich you wouldnt be able to skate (melting the ice by applying pressure and skating on a film of water)
Last edited by deuce_454; 01-15-2009 at 03:13 PM.
#13
TECH Apprentice
Join Date: Nov 2006
Location: Copenhagen
Posts: 369
Likes: 0
Received 0 Likes
on
0 Posts



